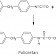
What is Distillation and What are the Methods?
Distillation is a fundamental and effective separation technique used in various industries, chemistry, and engineering to separate mixtures. It involves separating the components of a liquid mixture based on their different boiling points. But which distillation method is used when? In this article, we will explore distillation systems and discover the working principles, applications, and advantages of each.

There are four main types of distillation methods:
-
Simple distillation
-
Fractional distillation
-
Steam distillation
-
Vacuum distillation
1. Simple Distillation:
Simple distillation, the most basic distillation method, is used to separate two liquids with sufficiently different boiling points. In this method, the mixture is heated with a heat source, and the vaporized liquid is condensed and collected by a condenser.
Applications:
-
Production of alcoholic beverages like ethanol and water
-
Purification processes at laboratory scale
-
Homemade perfumes and essential oils
Advantages:
-
Easy setup and application
-
Low cost
Disadvantages:
-
Inefficient in separating liquids with close boiling points
-
May have low yield
2. Fractional Distillation:
Fractional distillation is used to separate liquid mixtures with close boiling points. In this method, the mixture is placed in a distillation column and heated gradually at different temperatures. At each temperature, the liquid with a different boiling point vaporizes and is collected in a separate container.
Applications:
-
Separation of petroleum fractions (gasoline, diesel, kerosene, etc.)
-
Purification of organic chemicals
-
Production of perfumes and essential oils
Advantages:
-
Effective in separating complex mixtures
-
High yield
Disadvantages:
-
More complex setup compared to simple distillation
-
Requires more time and energy
3. Steam Distillation:
Steam distillation is used to separate heat-sensitive or high-boiling-point liquids. In this method, steam is added to the bottom of the mixture, and the vaporized liquid is condensed and collected by a condenser.
Applications:
-
Purification of heat-sensitive vitamins and medicines
-
Production of glycerol and fatty acids
-
Extraction of flavors and fragrances in the food industry
Advantages:
-
Does not damage heat-sensitive liquids
-
Effective in separating high-boiling-point liquids
Disadvantages:
-
Requires additional equipment such as a steam generator
-
More complex setup compared to simple distillation
4. Vacuum Distillation:
Vacuum distillation is a distillation method carried out under low pressure. In this method, the pressure above the mixture is reduced, allowing for boiling at lower temperatures. This minimizes damage to heat-sensitive liquids and enables evaporation even under vacuum.
Applications:
-
Purification of high molecular weight polymers
-
Production of heat-sensitive medicines and chemicals
-
Separation of petroleum fractions (vacuum gas oil, etc.)
Advantages:
-
Does not damage heat-sensitive liquids
-
Allows distillation at low temperatures
-
Effective in separating liquids that can evaporate even under vacuum
Disadvantages:
-
Requires additional equipment such as a vacuum pump
-
More complex setup compared to simple distillation

Writer:
Professor Doctor Mustafa Yaşar
Industrial Design Engineer